In severely preterm newborns, biomarkers that signal early brain injury have been discovered by researchers.
The danger of brain injury in extremely preterm newborns is extremely significant. Researchers have discovered potential targets for the early therapy of such damage that are not located in the brain: Bacteria in the stomach of preterm newborns may play an important role in their development. Researchers discovered that overgrowth of the gastrointestinal tract with the bacterium Klebsiella is connected with an increase in the presence of particular immune cells as well as the development of neurological impairment in premature neonates, according to their findings.
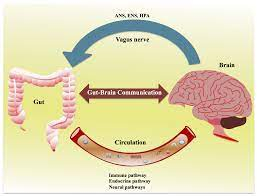
The gut-immune-brain axis is involved in a complex interplay.
A close relationship exists between the early development of one’s gut, one’s brain, and one’s immune system. This is referred to as the gut-immune-brain axis by researchers. It is the immune system’s job to work with the gut bacteria, which in turn helps the immune system to monitor gut microbes and create appropriate responses to them. In addition, the gut communicates with the brain through the vagus nerve as well as through the immune system to perform its functions. “We looked at the role that this axis plays in the development of the brain in very preterm infants,” explains David Seki, the study’s first author. “In healthy people, the microorganisms of the gut microbiome — which is a vital collection of hundreds of species of bacteria, fungi, viruses, and other microbes — are in a state of equilibrium. The gut microbiome is a vital collection of hundreds of species of bacteria, fungi, viruses, and other microbes. However, alterations are highly likely to occur, particularly in premature babies, whose immune system and microbiome have not had the opportunity to fully develop. These alterations may have deleterious consequences for the brain, such as memory loss “The microbiologist and immunologist provide further explanation.
Brain injury can be detected by looking for patterns in the microbiota.
According to David Berry, microbiologist and head of the research group at the Centre for Microbiology and Environmental Systems Science (CMESS) at the University of Vienna, as well as Operational Director of the Joint Microbiome Facility, “We have been able to identify certain patterns in the microbiome and immune response that are clearly linked to the progression and severity of brain injury,” he continues. “Importantly, such patterns frequently appear before any changes in the brain take place. This shows that there is a vital window of time during which brain damage in extremely preterm children can be prevented from progressing, if not completely avoided.”
The development of extremely preterm newborns has been the subject of extensive research.
The biomarkers that the interdisciplinary team was able to find serve as launching pads for the creation of relevant medicines. Lukas Wisgrill, a neonatologist from the Division of Neonatology, Pediatric Intensive Care Medicine, and Neuropediatrics at the Department of Pediatric and Adolescent Medicine at the Medical University of Vienna, explains that “our data show that excessive growth of the bacterium Klebsiella and the associated elevated??-T-cell levels can appear to exacerbate brain damage.” It was possible to identify these trends because, for the first time, researchers looked closely at the development of a very particular group of babies’ gut microbiome, immune system, and brain, as well as how these three systems interacted during the development process, says the researcher. Premature children born before 28 weeks of pregnancy and weighing less than 1 kilogramme were observed for several weeks or even months as part of the study. In addition to using cutting-edge methods (for example, 16S rRNA gene sequencing to examine the microbiome), the researchers analysed blood and stool samples, brain wave recordings (for example, aEEG), and magnetic resonance imaging (MRI) images of the infants’ brains to discover what was going on inside their heads.
Two new experiments are being conducted as part of the ongoing research.
An inter-university cluster project led by Angelika Berger (Medical University of Vienna) and David Berry (University of Vienna), the study serves as the starting point for a larger research project that will examine the microbiome and its significance for the neurological development of prematurely born children in greater depth. In addition, the researchers will continue to monitor the progress of the youngsters who participated in the initial trial. “It takes several years for the development of the children’s motoric and cognitive skills to become visible,” explains Angelika Berger. “Our goal is to gain a better understanding of how this very early development of the gut-immune-brain axis plays out over time.” The following are the most key collaboration partners for the project who have already signed on: “The parents of the children have shown a great deal of curiosity and openness in the study,” says David Seki. “The fact that we were able to get these critical insights is the sole explanation, in the end. We are quite grateful for your assistance.”
For More Information Visit : Theny Magazine





